Traditional Water Quality Indicators
Traditional Water Quality Indicators
Measuring chemical variables has been the traditional approach to water quality assessments. A few common chemical parameters for investigating water quality conditions are found below.
Common Chemical Water Quality Indicators
| Variable | Description | Example of Cause/Impact |
|---|---|---|
Available Oxygen |
The amount of molecular oxygen dissolved in water is an important measure of habitat availability for aquatic organisms. Low levels of oxygen result from the introduction of organic waste pollution which increases the rate of eutrophication and decreases the suitability for aquatic animal life. Sources include: agricultural runoff, urban runoff, and wastewater treatment plants. |   |
pH |
pH is a unit that expresses the strength of a solution based on its acidic or basic properties. Aquatic organisms can only function in a particular range of pH, and become forced to relocate when the surrounding water changes. Pollution from burning fossil fuels increases the amounts of sulfur and nitrogen oxides introduced into the water, thereby increasing the overall acidity. |   |
Turbidity |
The amount of suspended material in water can be measured by collecting the solids or assessing the relative light transmission of the suspension. The increased opaqueness is caused by increased sediment which negatively affect many aquatic organisms. Both algal production and fish reproduction and feeding can become diminished and some organisms, like shell-fish (continual filter-feeders) can become choked by sediment and eventually die in heavily turbid waters. |  |
Toxic Organic Compounds |
There are many chemicals that have the capacity to travel throughout a waterway. Many of these are pollutants and can cause significant distress to the surrounding habitat. Solutions such as oil or antifreeze enter a watershed from nearby runoff sources and directly poison the surrounding aquatic environment. With appropriate riparian vegetation, large surge concentrations of these chemicals can be prevented from directly entering the water. |  |
Heavy Metals |
Industrial effluents are major sources of heavy metals, and aquatic environments are extremely sensitive to even the smallest concentrations of these materials. Serious abnormalities have been reported in many aquatic organisms. Arsenic and mercury are two common examples of heavy metals, but other similar substances and compounds can also have significant effects on an aquatic community. |  |
Nutrients |
Additional nutrients, such as phosphorus and nitrogen, are added to streams by many avenues, but primarily through human sewage, animal waste, fertilizers and erosion. This area of water quality monitoring is greatly affected by both urban and agricultural human practices. Runoff from any inadequately covered lands can increase these nutrient loads and result in eutrophication of the nearby aquatic habitat. |   |
Although these chemical variables are useful to monitor impacts, they only provide a short-term picture of water quality at a sampled site and each can only represent a portion of a complete assessment. A couple disadvantages of using only chemical indicators include:
- Effects of certain isolated chemicals in a laboratory setting can be dramatically different than it's interactive effects with other variables
- Chemical testing is extremely expensive and labor intensive and not practical for monitoring non-point pollution sites such as urban and agricultural run-off
Chemical testing is much more applicable for point source pollution where industrial contamination is suspected.
Agriculture

Animal waste, including manure and urinary waste can enter streams directly when livestock wade in and around the water. Animals also trample streambanks and damage fish habitat. Animal wastes deposited in waterbodies can accelerate eutrophication and contaminate water used for fishing, swimming, and drinking. Streambank fencing is one way to protect streams from this type of livestock damage (See best management practices).
Construction

Areas under construction are usually devoid of any surface vegetation and topsoil and remain bare for extended periods of time. This process allows for extremely high quantities of subsoil to runoff into surrounding stormwater systems where receiving waters eventually accumulate large sediment loads. This increase in sediment decreases the amount of light penetrating through the water and thereby decreases the diversity and productivity of aquatic organisms.
Farm Runoff

Crops planted on the edge of streams can create many problems with soil stability. With heavy farm machinery for planting and harvesting and heavy rainfall, increased soil compaction and bank erosion will result over time and the soil will be pushed into the stream thereby increasing sediment load and decreasing the area of the bank. Planting close to a stream edge should be avoided; area around the stream should be maintained with adequate trees and vegetation along the bank (See best management practices).
Metals
Measures of Toxic Metals in Water
Heavy Metals
Toxic metals can be present in industrial, municipal, and urban runoff, and by definition, are harmful to humans and aquatic biota. Increased urbanization and industrialization have increased the levels of trace metals, especially heavy metals, in water ways. There are over 50 elements that can be classified as heavy metals, but only 17 that are considered to be both very toxic and relatively accessible. Mercury, lead, arsenic, cadmium, selenium, copper, zinc, nickel, and chromium, however, should be given particular attention in terms of water pollution and runoff/discharge effects. Toxicity levels depend on the type of metal, it's biological role, and the type of organisms that are exposed to it.Availability of Heavy Metals
Toxic metals are often added to the streams as salts (sulfides, phosphates and carbonates), are very insoluble in hard waters and usually travel with sediment. The transformation into readily accessible materials (for organisms) is a complex process and depends on many factors such as pH, sediment presence, and hardness. The availability of these metals is determined by precipitation-dissolution reactions which are strongly affected by pH. Therefore, at a lower pH, metallic ions (heavy metals in general) are more available and more reactive. Many of these metals then undergo methylation, as a result of bioaccumulation where bacteria absorb these elements and convert them from a metallic state into a toxic organometallic state. By becoming incorporated with an organic component, these metals become readily available to the first trophic level of the food chain and eventually lead to biological magnification throughout the system.Testing Information
Since even low concentrations of heavy metals can cause serious harm to aquatic ecosystems, very sensitive and precise instruments are required to appropriately measure these substances in water samples. Technically speaking, a measure of the availability of heavy metals to the first trophic level of organisms can made by taking the fraction extracted by a hydroxylamine hydrochloride reagent or by a chelating cation exchange resin.
Nutrients
Measures of Nutrients in Water
Availability of Nitrogen
Nutrients are elements (or substances) that are necessary for plant growth. Both nitrogen and phosphorus are two such elements, but large amounts of these elements in water systems can become burdensome by promoting excessive aquatic algal growth. Prime nitrogen sources, eroded soils and sewage sludge, are found in suspended sediments and range from 0.02 to 10%. These concentrations can exist in a variety of forms: organic N (dissolved or particulate), ammonia (dissolved or adsorbed on sediments), nitrate and nitrite N, and dissolved nitrogen gas (N2). Available N is the fraction of the total N that can be readily assimilated by either macrophytes or phytoplankton.Availability of Phosphorus
Phosphorus is essential to all life, but is naturally scarce in most systems. This element is added to stream systems primarily as animal and human waste, detergents, fertilizers, and as a product of soil erosion. Phosphorus, found in two major forms, inorganic and organic, is readily removed from aquatic environments when fine-grained particles and organic phosphorus compounds settle in the water. Inorganic P (from fertilizers and detergents) can lead to an overload of nutrients, eutrophication. Organic P eventually breaks down into inorganic orthophosphates and becomes readily available to plants.Testing Information
There are many different laboratory techniques used to assess the quantity and type of nitrogen and phosphorus in water and sediment samples. These analyses are quite complex and require a knowledgeable technician and the ability to isolate the desired form of the investigated element.
Oxygen
Measures of Oxygen and Oxygen Demand
Dissolved Oxygen
Oxygen in water is available to the plants and animals that live there only if it is dissolved. Dissolved oxygen or DO can range in concentration from 0 to 14.6 parts per million in water. This is also equivalent to a weight-based measure, milligrams per liter (or mg/l). The amount of oxygen that can be dissolved in water is inversely related to temperature - that is as the water temperature gets higher, the amount of oxygen that can be dissolved in the water goes down. It is also possible under some circumstances to have oxygen levels above 14.6 mg/l. This can happen where water goes over a dam or other structure that causes unusual amounts of mixing. The more oxygen that is in the water, the more diversity can be expected in the plants and animals found in the water.
Pollutants that make DO go down (besides heat) are any organic wastes such as animal or human sewage or any chemicals that will be decomposed by bacteria in the water. The growing bacteria that break down either the organic or chemical wastes consume oxygen for their reproduction and thus take oxygen out of the water and away from the other plants and animals.
Biochemical Oxygen Demand (BOD)
BOD is a lab test that measures the total amount of oxygen per unit volume of water required to bacterially oxidize (stabilize or break-down) the organic matter in the water. Samples are incubated under standard conditions for periods of 5, 10, 20 or 30 days. The standard test is for 5 days. The higher the BOD, the more oxygen depletion will take place.
Chemical Oxygen Demand (COD)
A similar test which measures how much oxygen that the oxidation of chemicals in the water might use.
Testing Information
Tests for these variables can be biased by the presence of toxic chemicals which kill the bacteria that would break down the organic materials or oxidizable chemicals. When measuring DO, care must be taken not to introduce extra air from the atmosphere as the sample is taken. All samples must be tested immediately or else iced down and taken back to the lab for testing. The ice slows down any on-going chemical and biological reactions.
Runoff
<
 |
Many pollutants enter nearby waterways by efficient movement across surfaces These surfaces do not allow for penetration and percolation which would normally occur in a well vegetated, open-soil area. Heavy concentrations of pollutants can enter a water system during storm events where a large amounts of water flow across impervious areas and flushes the compounds into the local river or stream. Pollutant levels from non-point sources can be decreased by supplying adequate vegetative ground cover throughout the riparian area (See best management practices).
Toxic Compounds
Measures of Toxic Organic Compounds
Availability of Organic Compounds
The focus of water quality attention in terms of toxic organic compounds is primarily given to manufactured organic chemicals applied to the surrounding land use as pesticides and herbicides (examples include DDT and PCBs). The availability of organic compounds to aquatic organisms in a water system is determined mainly by the rate of adsorption-desorption processes. These reactions can be affected by water quality factors including temperature, pH, and the presence/absence of other compounds. Concern should be given to the potential accumulation in the surrounding environment in terms of biomagnification of pesticides and PCBs - polychlorinated biphenyls.Testing Information
Different testing procedures are required to identify trace amounts of toxic organics such as chlorinated hydrocarbons which originate from agricultural runoff and industrial effluents. Special techniques for proper identification of oil residues in water is also necessary for a comprehensive analysis.
Turbidity
Measures of Turbidity in Water
Turbidity and Suspended Sediment
Sediment from nonpoint sources is the most widespread pollutant of surface water. Turbidity is the measure of the amount of suspended material in water and is determined by the relative light transmission of the suspension. Turbidity is an important consideration because it greatly reduces algal populations by inhibiting sunlight and slowing photosynthesis, changes heat radiation, has harmful effects on benthic fish and plants, and compromises most of water's major beneficial uses. The concentrations of suspended sediment in streams can be highly variable and are influenced by many factors, including the following: rainfall intensity and duration, soil condition, geology, topography, and present vegetation. Concentrations are measured in milligrams per liter (mg/l)
Testing Information
There are two typical scales used to measure turbidity, percent transmission and optical density. Percent transmission, or transmittance, varies from 0-100% and is based on the amount of light that is able to penetrate through the water sample. Optical density, or absorbance, is based on a logarithmic scale ranging from 2-0 where 2 represents the most turbid and 0 represents the least. A transmittance value of 50% corresponds to about a 0.3 absorbance value.
Waste Water Treatment

A wastewater treatment plant is where sewage goes from individual households and businesses. It removes a large percentage of the organic wastes (BOD) and sediments from the sewage and then discharges them into a local stream. Even so, the volume of the discharge can still cause a major pollution problem. You can find out more about what is in a wastewater sewage treatment plant at https://en.wikipedia.org/wiki/Sewage_treatment
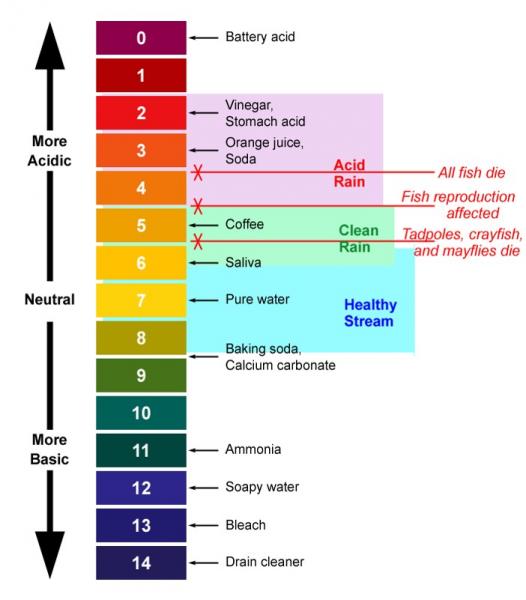
pH
pH and Water Quality
pH measures the acidity of the water. Thescale goes from 0 to 14 with 7 being neutral and numbers below 7 representing additional acidty and numbers above the lack of acid or "basic" conditions. Most organisms thrive in water that is near neutral.
As the amount of acidity increases, the impacts on aquatic life also increase. The acidity is toxic to aquatic life, contributes to the release of toxic metals into solution, and weakens the shells and skeletons of biota.
Major sources of acidity are industrial wastes and deposition of acids from air pollution (acid rain), The figure below shows some common items and their place on the pH scale. It also shows the levels at which various biota are impacted by acidity.