Cleveland Clinic team simulates cartilage response to loading
Columbus, Ohio (July 24, 2012) - A Cleveland Clinic research team is developing virtual models of human knee joints to better understand how tissues and their individual cells react to heavy loads – virtual models that someday can be used to understand damage mechanisms caused by the aging process or debilitating diseases, such as osteoarthritis.
 |
| Ahmet Erdemir, Ph.D. |
Led by Ahmet Erdemir, Ph.D., the team is leveraging the powerful computing systems of the Ohio Supercomputer Center to develop state-of-the-art computational representations of the human body to understand how movement patterns and loads on the joints deform the surrounding tissues and cells. Erdemir is the director of the Computational Biomodeling Core (CoBi) and a faculty member in the Department of Biomedical Engineering at the Lerner Research Institute (LRI) in Cleveland, Ohio.
“The aging process and debilitating diseases affect many aspects of the mechanical function of the human body: from the way we move to how our muscles, joints, tissues, and cells accommodate the loading exerted on the body during daily activities,” Erdemir explained. “Computational modeling techniques provide an avenue to obtain additional insights about mechanics at various spatial scales.”
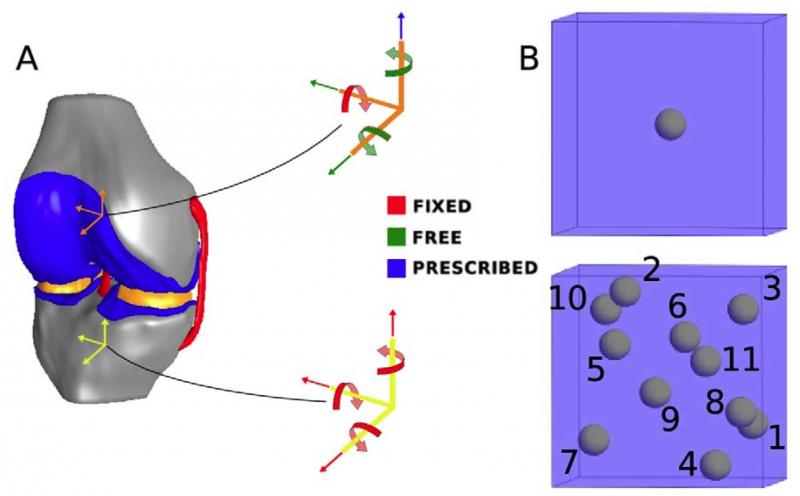
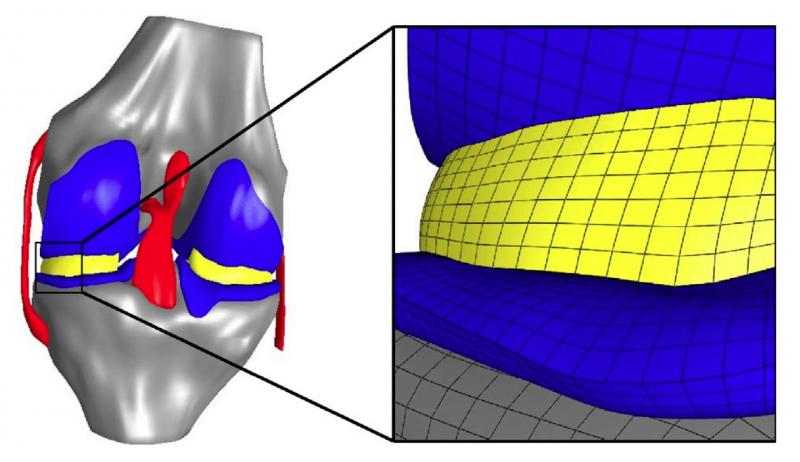
Many macro-scale studies have looked at how the various components of a knee joint – cartilage, menisci, ligaments and bone – respond to weight and other external loads. However, Erdemir and colleague Scott C. Sibole wanted to better understand how those large mechanical forces correspond to the related deformation of individual cartilage cells – or chondrocytes – within the knee. Previous micro-scale studies of cartilage have not commonly been based on data from body-level scales, in particular, by the musculoskeletal mechanics of the knee joint.
In addition, calculated deformations typically have been for a single cell at the center of a 100-cubic-micrometer block of simulated tissue; Erdemir used an anatomically based representation that calculated deformations for 11 cells distributed within the same volume.
“In both micro-scale approaches, the cartilage cells experienced amplified deformations compared to those at the macro-scale, predicted by simulating the compression of tissues in the knee joint under the weight of the body,” Erdemir found. “In the 11-cell case, all cells experienced less deformation than the single cell case, and also exhibited a larger variance in deformation compared to other cells residing in the same block.”
Erdemir’s method proved to be highly scalable because of micro-scale model independence that allowed exploitation of distributed memory computing architecture. As a result, Sibole, a research engineer at LRI, was able to leverage the computational muscle of OSC’s IBM 1350 Glenn Cluster. At the time, the 9,500 nodes of the Glenn Cluster provided 75 teraflops of computing power, tech-speak for 75 trillion calculations per second. Recently, the Glenn Cluster was partially decommissioned when engineers deployed the center’s more powerful HP-Intel Xeon Oakley Cluster.
“Both of OSC’s two most recent flagship computing systems were specifically designed to support biomedical applications, such as those employed by Dr. Erdemir and Mr. Sibole,” said Ashok Krishnamurthy, OSC interim co-executive director. “Researchers working at Ohio’s various respected medical centers are conducting an ever-increasing load of computational studies and analyses, and they now represent a significant share of our user community.”
An article authored by Erdemir and Sibole, “Chondrocyte Deformations as a Function of Tibiofemoral Joint Loading Predicted by a Generalized High-Throughput Pipeline of Multi-Scale Simulations,” was recently published in PLoS ONE, an international, peer-reviewed, open-access, online journal. Grant funding from the National Institute of Biomedical Imaging and Bioengineering, National Institutes of Health supported the study.
The Ohio Supercomputer Center (OSC), a member of the Ohio Technology Consortium of the Ohio Board of Regents, addresses the rising computational demands of academic and industrial research communities by providing a robust shared infrastructure and proven expertise in advanced modeling, simulation and analysis. OSC empowers scientists with the vital resources essential to make extraordinary discoveries and innovations, partners with businesses and industry to leverage computational science as a competitive force in the global knowledge economy, and leads efforts to equip the workforce with the key technology skills required to secure 21st century jobs. For more, visit www.osc.edu.
The Department of Biomedical Engineering (BME) at the Lerner Research Institute is committed to investigation, innovation, and translation of scientific discoveries to enhance patient care. The Lerner Research Institute is home to Cleveland Clinic's laboratory-based, translational and clinical research. For more, visit www.lerner.ccf.org/bme.