The stalwart computing power of the Ohio Supercomputer Center recently played an integral role in a groundbreaking discovery by Cornell University scientists.
By combining theory and computational modeling, the researchers predicted that the lightest known metals in the universe, lithium (Li) and beryllium (Be), will bond under high levels of pressure and form stable — and possibly superconductive — alloys.
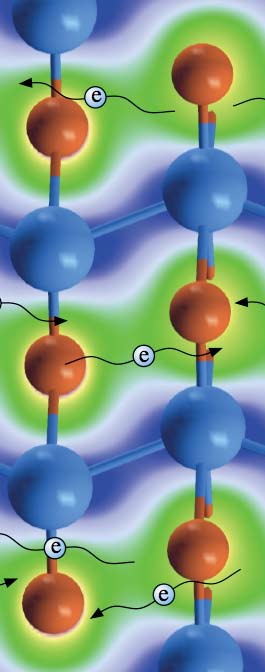
Under normal conditions, Li and Be repel each other. Unexpectedly, they also found that the combination creates a quasi two-dimensional electron gas sandwiched between the Li layers of the LiBe alloy.
“While I searched for stable high-pressure structures using the random search method, my colleague used chemical information to determine likely stable bond arrangements for LiBe,” said Richard Hennig, Ph.D., a Cornell University professor in materials science and engineering. “OSC’s queuing system enabled us to simultaneously run large numbers of density functional calculations within a few weeks time. This is a great benefit over other supercomputing centers.”
The research, supported by the National Science Foundation, appeared in the Jan. 24, 2008, issue of the journal Nature.
“The Ohio Supercomputer Center was my first choice for computing the structures at each composition and pressure,” Dr. Hennig said. He previously used OSC extensively for ab-initio and quantum Monte Carlo calculations of defects in semiconductors and phase transformation in transition metal alloys as a post-doctorate researcher under Ohio State University Physics Professor John Wilkins. Their on-going collaborations grant Dr. Hennig access to OSC’s supercomputers from Cornell.
--
Project leads: Roald Hoffmann, Ph.D., & Richard Hennig, Ph.D., Cornell University
Research title: Emergent reduction of electronic state dimensionality in dense ordered Li-Be alloys
Funding sources: National Science Foundation & the Petroleum Research Fund of the American Chemical Society