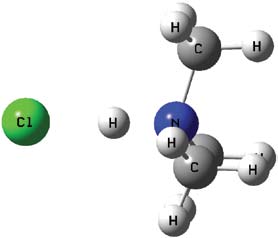
Hydrogen bonds are the ‘chemical glue’ that binds two molecules together and makes them. act as one. They are crucial in applications such as enzyme kinetics, DNA replication and the binding of drugs to specific targets in the body.
Janet E. Del Bene, Ph.D., professor emeritus of chemistry at Youngstown State University, has relied on the Ohio Supercomputer Center since its inception in 1987 for her research in quantum theoretical chemistry.
“As a computational chemist, my laboratory is the computer,” said Dr. Del Bene, an expert in hydrogen bonding. Hydrogen bonds are responsible for the properties of water. Because most chemical reactions occur in water, chemists need to understand how hydrogen bonds influence chemical reactions.
Dr. Del Bene has addressed questions concerning the stabilities of hydrogenbonded complexes, the methodological dependence of their computed properties, and has connected their infrared (IR) spectrosopic properties to the type of hydrogen bond present. Her work resolved what appeared to be a contradiction between theory and experiment in the description of certain types of hydrogen bonds.
Most recently, she has focused on how nuclear magnetic resonance (NMR) spectroscopy can be used to extract structural information about hydrogen bonds. By computing magnetic properties that can be measured experimentally in an NMR spectrum, Dr. Del Bene developed a way to characterize hydrogen bonds from their spectra and to obtain structural data for hydrogen-bonded systems in solution. Through her work, Dr. Del Bene and her colleagues have developed the chemical equivalent of infrared and nuclear magnetic resonance spectroscopic fingerprints of hydrogen bonds.
So what is the ultimate purpose of this work? Computational chemistry leads to a better understanding of chemistry and chemical reactions, and that understanding is the key to progress.
--
Project lead: Janet E. Del Bene, Ph.D.,Youngstown State University
Funding source: National Science Foundation, CHE-9873815