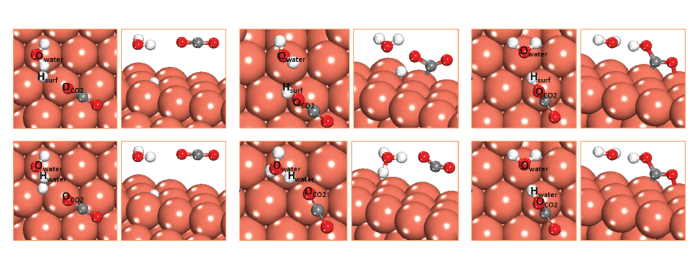
Optimized structures of the initial, transition, and final states associated with the elementary step of CO2 reduction to COOH with 1 H2O in the (a) water-solvated, and (b) water-assisted models. (Orange=copper, red=oxygen, gray=carbon, white=hydrogen.)
Carbon dioxide (CO2) is considered an atmospheric trace element, yet also is recognized as a greenhouse gas that has increased significantly since the advent of industrialization. The conversion of CO2 to alcohols is a potentially attractive way to translate intermittent sources of renewable energy, such as wind and solar, into a viable form of chemical energy for our existing transportation infrastructure. For this approach to be feasible, the CO2 conversion must be done efficiently and the fuel product selectivity optimized. Identifying such catalysts has been elusive, despite extensive experimental work over two decades.
As members of a DOE Energy Frontier Research Center project at Louisiana State University, Aravind Asthagiri, Ph.D., and his colleagues at The Ohio State University and Penn State University are examining why CO2 reduction on copper (Cu) electrodes produces methane and ethylene instead of methanol, as seen in heterogeneous methanol synthesis. Such work represents a first step in understanding the important factors that affect selectivity and activity of CO2 electroreduction catalysts. The research team leveraged Ohio Supercomputer Center resources to perform density functional theory (DFT) calculations to investigate both the thermodynamics and kinetics of CO2 reduction on Cu.
“Our calculations identified the free energies and activation barriers of the elementary steps for CO2 electrochemical reduction on copper electrodes,” said Asthagiri, an Ohio State associate professor of chemical and biomolecular engineering. “This approach represents a paradigm shift in computational electrocatalysis, as we are applying a novel method to evaluate potential-dependent activation barriers, which have thus far been rare in examining electrocatalytic reactions with DFT.”
The activation barriers turn out to be critical to understanding the unique selectivity found in Cu electrodes. Based on these new DFT calculations, the team determined a reaction path that is able to reconcile a range of experimental data on CO2, carbon monoxide (CO) and formaldehyde (CH2O) reduction on Cu electrodes. Instead of proceeding through a CHO intermediate (aldehyde, which leads to methanol), methane formation goes through reduction of CO to COH (alcohol), which eventually leads to CHX species (hydrocarbons) that can produce both methane and ethylene, as has been observed experimentally.
“This analysis suggests that future design of electrocatalysts for CO2 electrochemical reduction should focus on the relative energetics of COH versus CHO formation to tailor the selectivity of the desired products towards liquid fuels, such as methanol,” said Asthagiri.
Project Lead: Aravind Asthagiri, The Ohio State University
Research Title: Selectivity of CO2 reduction on copper electrodes: The role of the kinetics of elementary steps
Funding Source: U.S. Department of Energy