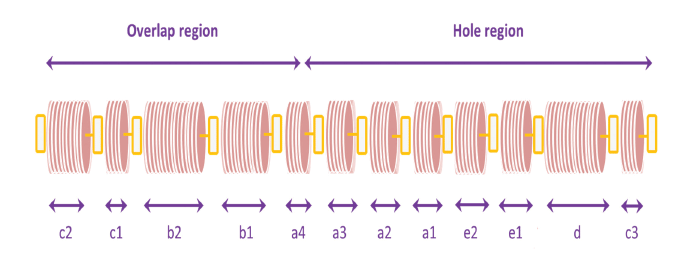
A series of light and dark bands is observed across the axis of the fibril when collagen molecules are stained with metal ions and then viewed in the electron microscope. The bands are designated sequentially c2–c3, as shown.
By investigating the mechanisms of bone formation, researchers at the University of Akron may help develop treatments for bone-related diseases such as osteomalacia, more commonly known as rickets, and osteogenesis imperfecta, a genetic disorder in which bones break easily.
Osteomalacia is not the same as osteoporosis, another bone disorder that can also lead to bone fractures. Osteomalacia results from a defect in the bone-building process, while osteoporosis develops because of a weakening of previously constructed bone.
Up to 50 percent of bone is made of a modified form of the mineral hydroxyapatite; the highly ordered structure includes insoluble protein, collagen, and is associated with other soluble proteins and small molecules such as citrate. The nucleation and growth of hydroxyapatite mineral crystals play an important role in controlling the physical properties of bone, and its source of calcium.
“My research group is interested the fundamental mechanisms of bone biomineralization from the molecular- to the micron-scale,” said Nita Sahai, an Ohio research scholar and professor of polymer science at the University of Akron.
The mechanisms for bone mineralization are not clearly known so far; a major limitation to progress in this research area is the inability to sufficiently sample the bone’s nucleation phenomena at the Angstrom (0.1 nanometer) to nanometer length-scale and microsecond time-scale. This hurdle exists because regular molecular dynamics simulations cannot accurately sample the calcium and phosphate ion distributions in the nucleating cluster.
To overcome these limitations, Sahai and her team are using the Ohio Supercomputer Center to calculate the ion behavior in the presence of collagen fibril by using Hamiltonian Replica-Exchange Molecular Dynamics. (See illustration above.) These advanced computer simulations allow them to determine the mineralization mechanisms across a wide range of length-scales and time-scales, approaching 100 nanoseconds. This compares drastically to the current standard, which is one to a few nanoseconds. Experimental results obtained in the laboratory provide benchmarks for the computational results.
“By analyzing the computational results, we can establish detailed mechanisms for how bone forms,” Sahai said. “Not only does this represent a major advancement in the methodology for modeling biomineralization mechanisms, it will contribute to scientists designing drugs for treating debilitating bone diseases.”
Project Lead: Nita Sahai, University of Akron
Research Title: Computational approach to bone biomineralization mechanisms: molecular-to-micron-scale hierarchical structure.
Funding Source: University of Akron
Website: http://www.uakron.edu/sahai-group