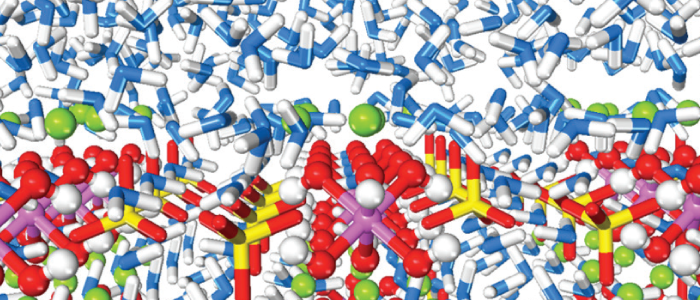
Above: In order to understand and design corrosion-resistant alloys, Heinz conducted atomistic simulation, guided by experimental results, to determine phase changes upon heating, oxidation, and aqueous corrosion.
Scientists at the University of Akron, in collaboration with partners at UCLA, are investigating the unique properties of metal alloy nanostructures – materials measuring 1-1000 nanometers in length – that have potential applications in the manufacture of fuel cells, batteries, automotive catalysts, sensors and nanoeletronic devices.
More specifically, the research delves into modeling alloy catalysts that drive the electricity-generating chemical reactions within fuel cells by employing smaller amounts of the expensive metal platinum, which could accelerate the world’s transition to emissions-free passenger vehicles and mobile power generation. Recently, it has been discovered that the reactions can be greatly enhanced and the costs lowered by introducing cheaper metals, such as iron, cobalt, nickel, copper, chromium or maganese, into the platinum.
Further, the research group believes that highly accurate synthesis, characterization and computation tools to predict internal atomic structures and the chemical properties of alloy nanoparticles at realistic length scales also can be applied to improve magnetic information storage, biomarkers and catalysts for other reactions.
“The performance space of nanoalloys is exponentially higher compared to pure-metal nanostructures from routine synthesis methods,” explained Hendrik Heinz, Ph.D., associate professor of Polymer Engineering at the University of Akron. “By accessing Ohio Supercomputer Center systems to create multi-scale simulations and develop force fields of revolutionary accuracy at length scales of 1 to 100 nanometers, we aim to close the wide gap between experimental capabilities and missing theoretical understanding of growth mechanisms, shape control and catalytic performance.”
The coordinates of all atoms in the synthesized nanostructures will be monitored using a powerful electron microscope to feed computer models, validate interatomic potentials and predict 3-D atomic ordering and chemical reaction rates. To improve catalytic properties, the researchers are closely connecting the unique chemistry challenges of alloy synthesis and testing, characterization challenges to resolve 3-D atomic structures and annealing transitions, and computational challenges in multi-scale simulations and development of models. The outcome will be a full understanding of alloy nucleation and growth, atomic ordering and quantitative engineering of the reactivity from first principles.
Ultimately, the three research groups in this collaborative effort hope to achieve a full understanding of alloy nucleation and growth, atomic ordering and quantitative engineering of the reactivity from first principles and to share insights into new force fields and computational algorithms by distributing them as open-source data through research websites and software repositories.
Project Lead: David A. Drabold, Ph.D., Ohio University
Research Title: Simulations of complex chalcogenide and oxide materials
Funding Source: National Science Foundation, Army Research Office
Website: http://www.phy.ohiou.edu/~drabold/