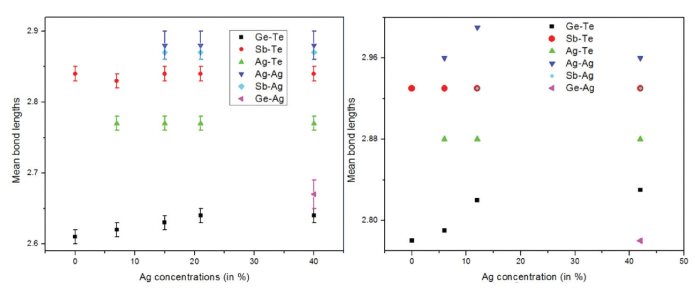
Above: Drabold’s group is performing the first simulations of GeSbTe:Ag materials – GST doped with silver. The charts above provide a direct comparison between the coordination statistics found in experiment (top) and theory (bottom).
A research group at Ohio University has been studying the physics of chemical elements in the oxygen family that lack a crystalline structure, elements known as amorphous chalcogenide materials. Chalcogenide materials containing traces of silver – a state referred to as “doped” – make rigid, fast-ion conductors, useful in applications such as solid oxide fuel cells and as a novel computer memory material. They are also working on thermal imaging sensors used in “night vision” applications.
The OU group analyzed amorphous titanium dioxide (TiO2), revealing the microscopic structure – essentially a chemically ordered continuous random network, but with interesting twists such as the existence of two distinct local environments for titanium. Furthermore, while amorphous TiO2 has well known photo-catalytic properties in nanophase form, the group is looking for similar characteristics from the amorphous phases of the material.
The researchers also are looking at two prime candidates for sensors and memory components: germanium-selenide (GeSe) and germanium-sulfide (GeS) hosts doped with up to about 40 percent of silver (Ag).
“Experimentally, it is known that the GeSe system has significantly better ionic mobility than the sulfide, which is interesting and unexplained,” explained David Drabold, Edwin and Ruth Kennedy Distinguished Professor of Physics at Ohio University. “We have shown that, despite the great similarities in S and Se chemistry, the Ag tends to be trapped in more restricted environments in the sulfide.”
The group also is investigating chalcogenide-based “phase-change memory,” which at least one telephone manufacturer has begun to deploy in some of its cell phones. Here, they’ve found that crystalline germanium-antimony-tellurium (GeSbTe) alloys have a much higher electrical conductivity than those in amorphous states, forming the basis of on/off contrast required for memory applications.
“In a speculative but potentially important line of research, we have begun to use our Ohio Supercomputer Center resources to look at a system that is essentially a hybrid of the two types of chalcogenide memory materials: we are performing the first simulations of GeSbTe:Ag materials – GST with silver,” Drabold said.
The group has learned that these materials maintain their semiconducting character with up to 50 percent Ag content. They are studying the topology of the network and silver dynamics, as well as the peculiar ability of the system to maintain its semiconducting character to very high metal concentration.
Project Lead: David A. Drabold, Ph.D., Ohio University
Research Title: Simulations of complex chalcogenide and oxide materials
Funding Source: National Science Foundation, Army Research Office
Website: http://www.phy.ohiou.edu/~drabold/