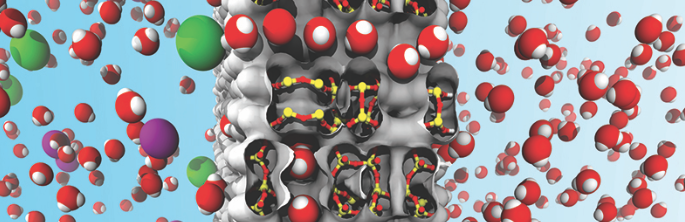
Over 96 percent of the water on Earth is undrinkable and unusable for most human purposes. While removing salt from ocean water is possible, desalinated water costs up to ten times more than typical groundwater. Li-Chiang Lin and his team at The Ohio State University’s chemical and biomolecular engineering department, with collaborators, are working to bring these costs down by identifying ideal water desalination materials. They have narrowed down candidates by using molecular simulations through the Ohio Supercomputer Center and identified promising ultrathin-film membrane candidates for water desalination to provide maximum water fluxes while blocking any salts from coming through.
“The role of the supercomputer over here is, you know, it allows us to study a number of materials efficiently to explore promising ones,” Lin said. “We perform molecular simulations, which also allows us see exactly how water molecules can permeate through a membrane and how salt ions are going to interact with the membrane.”
Lin’s group has identified two promising groups of materials as favorites for efficient water desalination. One is single-walled aluminosilicate nanotubes. These materials easily transport water due to their hydrophilic properties as well as straight and uniform channels. The group runs tests on nanotubes of varying pore sizes to find the right structure that will most efficiently trap salts while allowing a high degree of water flow through the membranes.
The other group is man-made, three-dimensional crystalline structures called zeolite materials. These structures can be made into an ultrathin film, down to a nano-scale thickness (so-called zeolite nanosheets), an excellent property for separations. Molecular simulations were carried out to model varying pore sizes, channel sizes and structure of different zeolite materials to identify their potential in water desalination.
“There are in fact millions of zeolite materials that may possibly exist so this is why we firstly studied 27, trying to create a guideline for the rational design of novel materials,” Lin said. “One million structures is a little bit too many to study.”
Lin’s studies have taken him around the country to places like UC Berkeley and the Massachusetts Institute of Technology where he used world-known state-of-the-art supercomputing clusters. He remains impressed by what OSC can offer researchers in Ohio.
“It has been an amazing experience to use OSC for research projects,” Lin said. “OSC offers excellent support and OSC’s supercomputers, in particular the newest machine Owens, is very fast. Additionally, the typical waiting time for jobs is relatively short. All of these have largely helped facilitate the progress of our projects, and we really appreciate all the resources provided from OSC.”
###
Written by Audrey Carson
Project Lead: Li-Chiang Lin, The Ohio State University
Research Title: Computational discovery of nanoporous materials for water desalination
Funding Source: The Ohio State University
Website: https://lin-group.engineering.osu.edu/people/lin.2645