Charis Eng, M.D., Ph.D., takes a gene-informed approach to personalized risk assessment and medical management of her patients and families. Her patient-focused research in genes, when altered, or mutated, associating with specific clinical features, such as cancer and autism spectrum disorder (ASD), provides the scientific evidence on which she practices precision medicine. Eng utilizes these insights as she evaluates the patients she sees at the Center for Personalized Genetic Healthcare (CPGH), the clinical arm of Cleveland Clinic’s Genomic Medicine Institute (GMI).
Eng first identified germline mutations in the cancer fighting gene, phosphatase and tensin homolog (PTEN), in Cowden syndrome patients in 1997. Since then, her study of PTEN has resulted in characterizing increased risk of cancers (e.g. breast, thyroid, endometrial, kidney, colorectal, melanoma) and autism (ASD). Yet, it remains unclear why mutations in one gene, PTEN, can result in ASD and/or cancer.
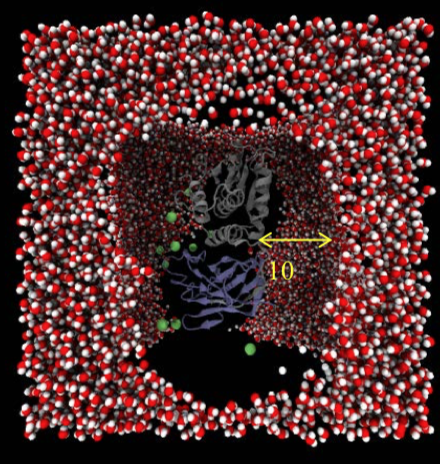
Recently, Eng’s lab turned to the Ohio Supercomputer Center (OSC) to use molecular dynamics (MD) simulations to gain atomic-level insight into the protein encoded by PTEN.
“Molecular dynamics simulation is an invaluable tool for investigating the behavior of proteins and to decipher the effects of mutation-induced changes in structure and function,” according to Eng. “Understanding the functional impact mutations have on the structure of PTEN will aid in the identification of specific mutations that contribute to ASD or cancer.”
In 2017, Iris Smith, Ph.D.—a postdoctoral fellow in Eng’s lab—used an OSC startup allocation to complete initial benchmarks for PTEN systems, which included about 76,000 atoms. Smith built each system and performed the simulations using GROMACS (GROningen Machine for Chemical Software) on the Oakley cluster, which significantly sped up the simulations previously run on an in-house system.
With the initial benchmarks complete, Smith plans to run larger simulations, analyzing hundreds of thousands of atoms. “These tools allow us to see [inside] how molecules are working and give us a much deeper understanding of the disease mechanism,” Smith says.
Images from simulations may someday be used to help physicians understand the structure and function of a particular mutation, enabling a personalized, gene-informed medical management plan, including appropriate screenings and early interventions for either cancer or ASD risk.
“Performing MD simulations at times can be far removed from patient care,” Smith said. “But we will utilize this to aid in the prediction of specific mutations, directly informing our patient care.”
###
Written by Ross Bishoff
Project Lead: Charis Eng, M.D., Ph.D., The Cleveland Clinic
Research Title: Insight into Structure and Dynamics from Computational Modeling and Simulation: The Effects of Germline PTEN Mutations in Autism Spectrum Disorder and Cancer
Funding Source: National Institute of Neurological Disorders and Stroke; National Cancer Institute; The Ambrose Monell Cancer Genomic Medical Clinical Fellowship.
Website: my.clevelandclinic.org/staff/6757-charis-eng#education-content