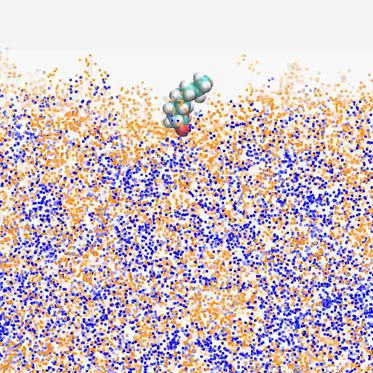
The way your favorite beverage tastes, how foamy your hand sanitizer is, the way certain products smell or feel or taste or flow is based on whether molecules in a water-alcohol solution go to the surface of the mixture or stay in a group.
Research into the behavior of molecular interfaces from a theoretical chemistry perspective has been fairly common over the years. However, researching these behaviors from an industrial point of view has been sparse. That’s where Case Western Reserve University’s Daniel Lacks, Ph.D., comes in.
Lacks, the chair of CWRU’s Department of Chemical and Biomolecular Engineering and a professor of chemical engineering, performs computational simulations through the Ohio Supercomputer Center to research the behavior of surfactants in water-alcohol mixtures to better understand, and in the future control, the characteristics of products we regularly consume.
A surfactant is a molecule that reduces surface tension at an interface—possibly between two liquids, a liquid and a gas or a liquid and a solid—and their presence alters the overall behavior of a product or system. Not only could Lacks’ research apply to personal care products or beverages, but also to biotechnology, with regard to the structure of proteins in the body.
“Originally we were looking at this in regard to personal care products,” Lacks said. “But there’s really interesting science behind it so we got an NSF (National Science Foundation) grant to study the general idea of solvation (the interaction of a solvent with dissolved molecules) and whether molecules go to a surface.”
Products interact with the environment at surfaces, so understanding those behaviors would allow us to manipulate a product’s taste, smell, or any number of characteristics a consumer may prefer.
Lacks performed “computationally intensive” simulations on OSC’s Owens and Oakley clusters in order to carefully control and validate experiments, artificially changing aspects of a molecule to determine how it will behave.
“With a mixture you need bigger systems,” Lacks said. “It takes a lot of averaging to get these small changes to be significantly visible, and to see small differences you need to run long simulations and that’s where we use OSC resources.
“The key is understanding what aspects of the molecule would lead to it going to the surface rather than staying in the bulk. That could help design new molecules.”
Project Lead: Daniel Lacks, Ph.D., Case Western Reserve University, Chair, Department of Chemical and Biomolecular Engineering C. Benson Branch Professor of Chemical Engineering
Research Title: Molecular Dynamics Simulation of Surfactants at Interfaces of Aqueous Solutions
Funding Source: National Science Foundation
Website: http://engineering.case.edu/profiles/djl15